Migraine Burden of Disease
Find information about migraine pathophysiology, phases, diagnosis, and more to help better identify and treat this disease
Watch this brief video for more information about the prevalence of medication overuse and its impact on migraine
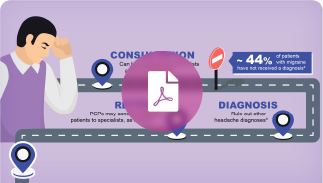
Download this resource for high-level data on the prevalence of migraine, and guidelines for measuring functional disability
Watch this comprehensive seminar detailing the key information you need about migraine burden, diagnosis, and treatment
Diagnosis and Management
Learn how to better differentiate migraine from other headache disorders in order to yield better patient outcomes
Find ICHD-3 criteria to help more accurately identify and diagnose patients suffering from migraine
Use this brief questionnaire to help screen your patients for migraine, based on the ID Migraine™ Validation Study
Learn the key differences between acute and preventive migraine treatment options, and what to consider when making treatment decisions
Download this guide to gain a deeper understanding of the patient migraine experience, and learn how to better help your patients manage their condition
Visit the Headache Journal website to see the AHS Consensus Statement with updated guidance on the use of preventive and acute treatments for migraine in adults
CGRP and Migraine Pathophysiology
Download this resource and gain a foundational understanding of CGRP and the role of CGRP signaling in migraine
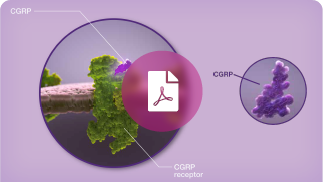
Find a high-level introduction to CGRP,
CGRP-R, and the trigeminovascular system
Listen in as Drs. Jerome Lisk and Merle Diamond discuss the role of CGRP in migraine and how approaches to targeting CGRP signaling impact our ability to treat
Clinical Study of Migraine
Watch this video to learn more about migraine clinical trials and the factors that impact study design
Pharmacovigilance
Download this resource to learn more about the process of adverse event detection in clinical trial and real-world settings
Learn the key differences between cumulative incidence and exposure-adjusted incidence rates, and see how each is derived
Use this interactive guide to learn how adverse events are detected, assessed, and understood in both clinical trials and real-world settings
Watch this video to learn more about adverse event detection and reporting
Watch this video to see the history of pharmacovigilance in the US and better understand key patient safety considerations in the clinical setting
Monoclonal Antibodies
Find out more about the use of SMDs and mAbs in managing neurologic conditions, and the key considerations when evaluating each
Watch this video to gain a better understanding of the effectiveness of monoclonal antibodies and small-molecule drugs for treatment
Download this resource and learn more about the defining characteristics of mAbs and SMDs